Study Details
Study Design
Prospective, multi-centre, open-label, single-armed, non-interventional observational clinical investigation.
Schematic
- Screening/Eligibility → All-comer patients (≥80 years) affected by acute coronary syndrome (NSTE-ACS), stable angina, or silent angina, who qualify for PCI according to ESC treatment guidelines and physicians’ clinical routine estimation.
- Follow-up → 6 months, and 12 months
- Endpoints
- Primary Endpoint:
DOCE (as per ARC-2) at 12 months, defined as composite of cardiovascular death, MI not clearly attributable to a non-target vessel and clinically driven TLR.
- Primary Endpoint:
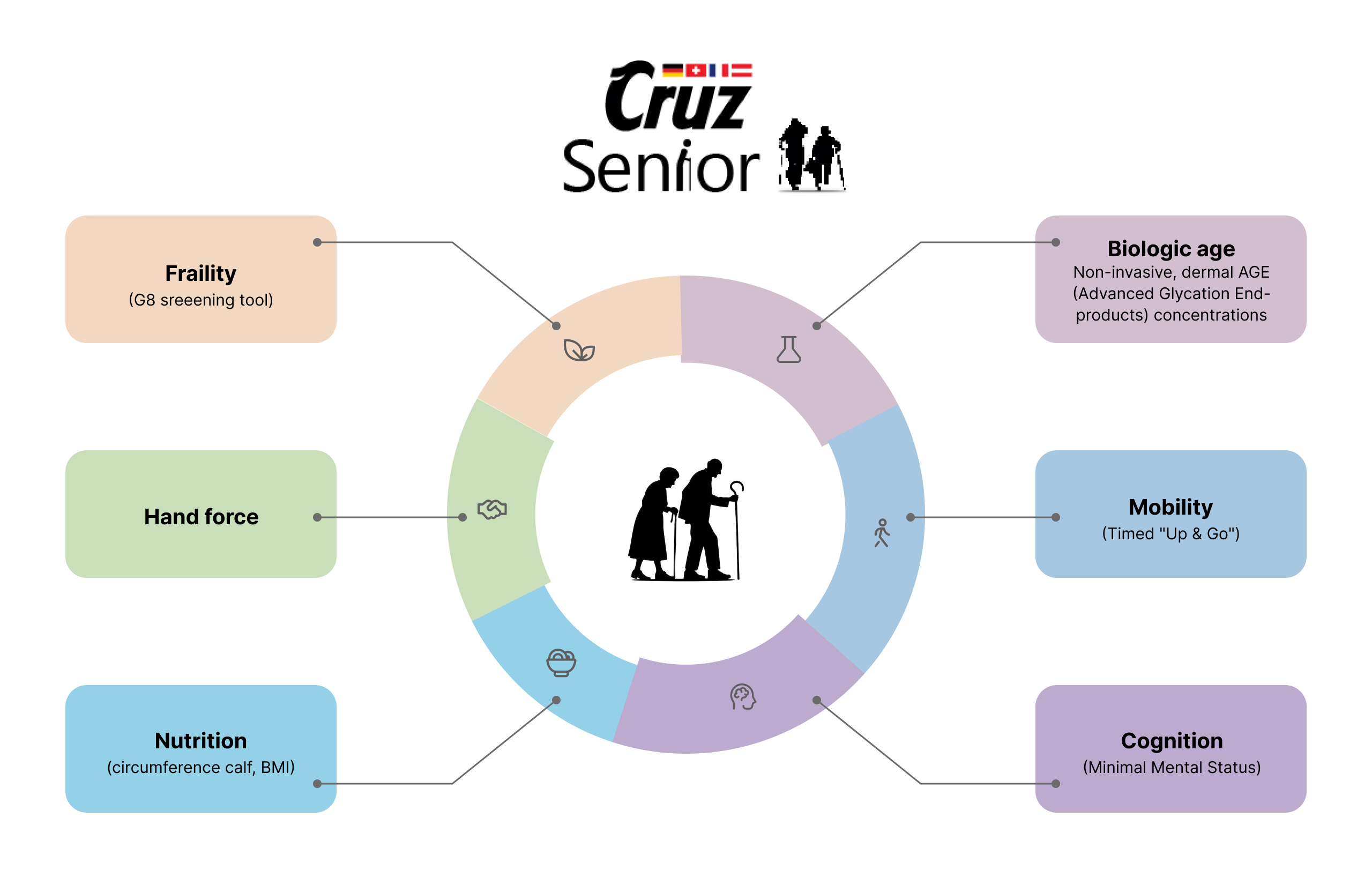
This study also focuses on Quality-of-life.
Comprehensive frailty assessment
Abbreviations: ACS: Acute Coronary Syndrome, NSTE-ACS: Non-ST-Elevation Acute Coronary Syndrome, PCI: Percutaneous Coronary Intervention, ESC: European Society of Cardiology, DOCE: Device Oriented Composite Endpoint, ARC2: Academic Research Consortium 2, MI: Myocardial Infarction, TLR: Target Lesion Revascularization