Trial Results
Primary endpoints at 12 months:
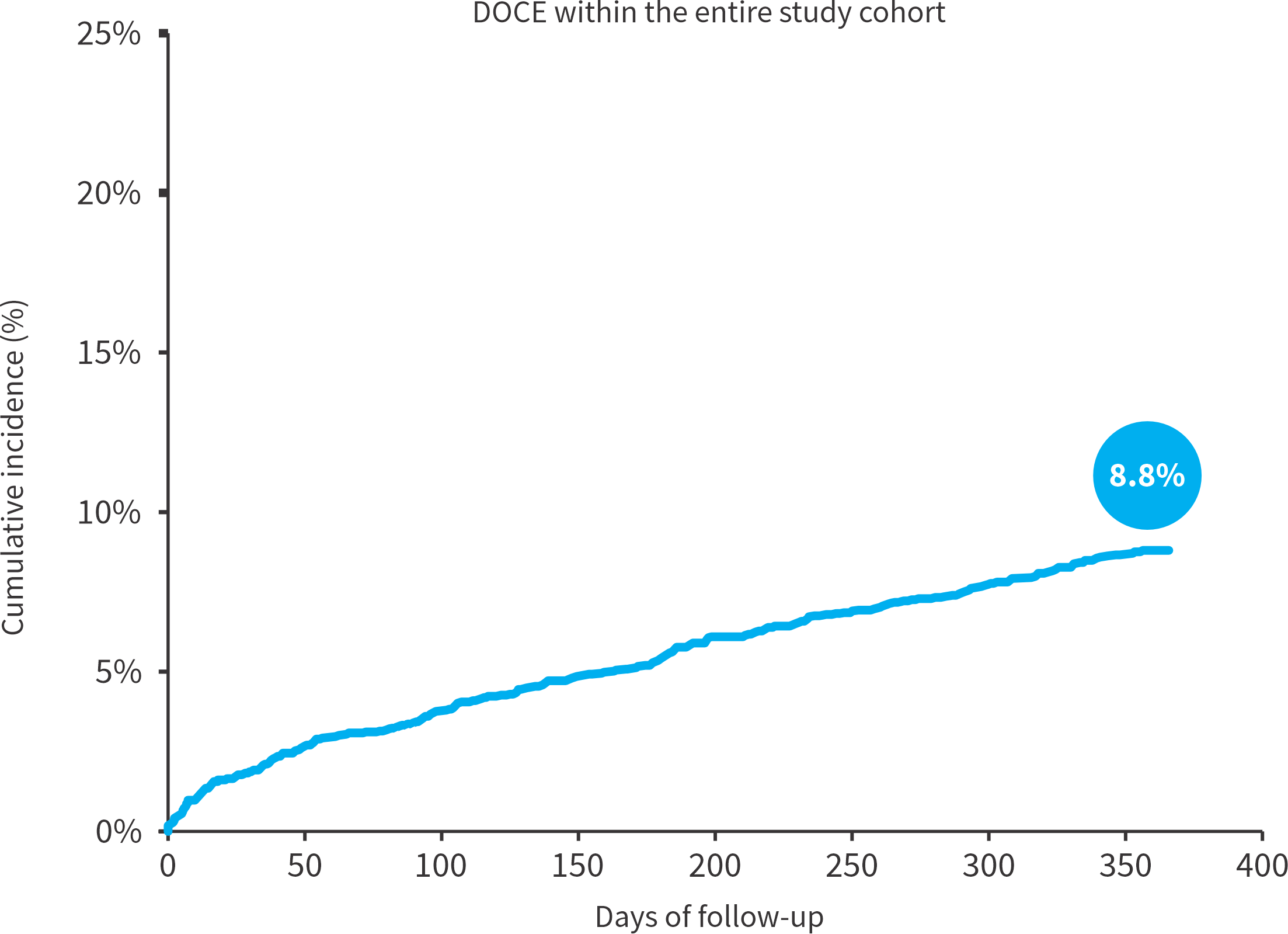
Device oriented primary endpoint (DOCE)
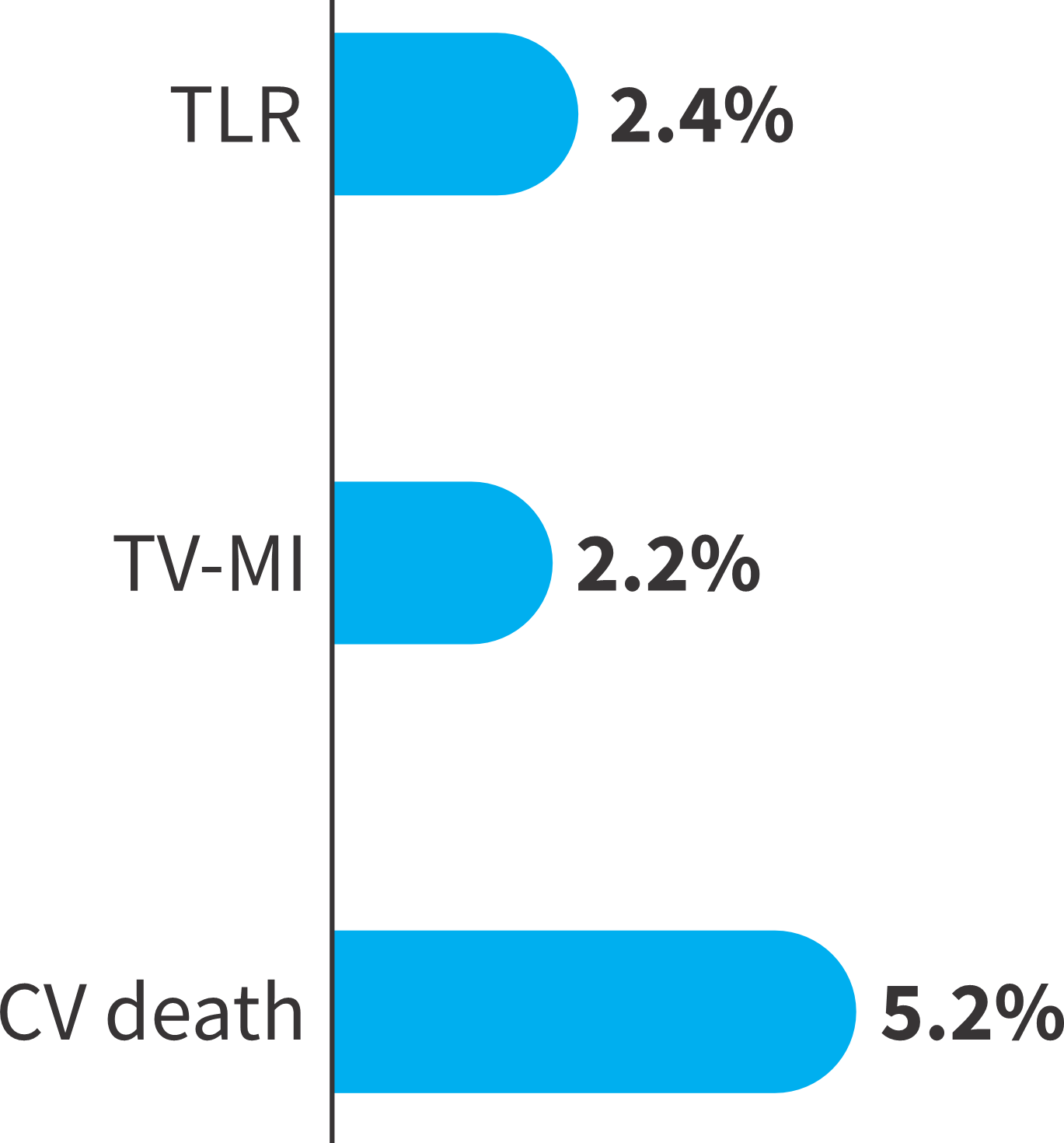
Individual components of the primary endpoint
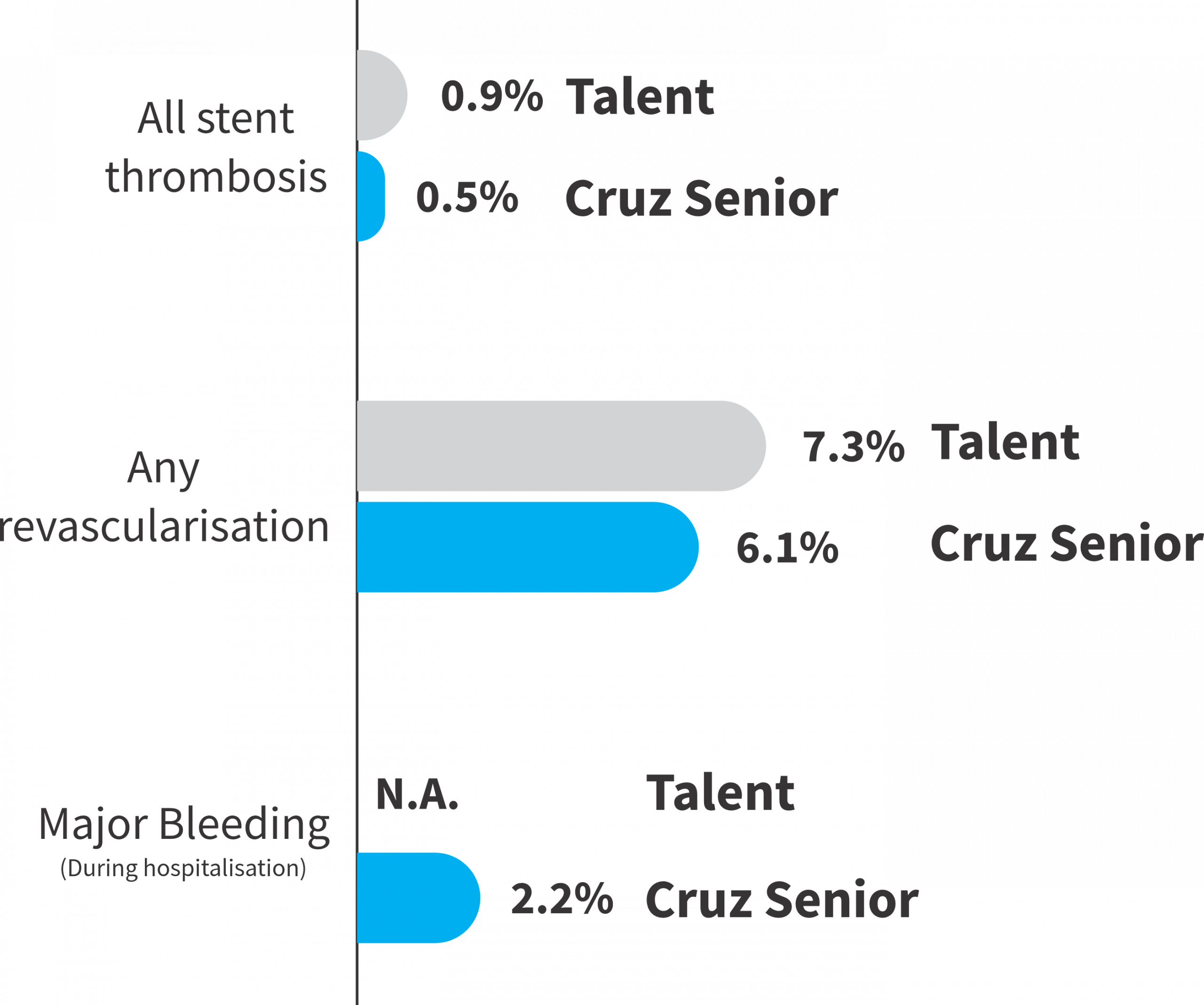
Thrombosis and Bleeding endpoints
Overall improvement in Patient Reported Outcome Measures (PROMs) at follow-up assessed by the Seattle Angina Questionaire
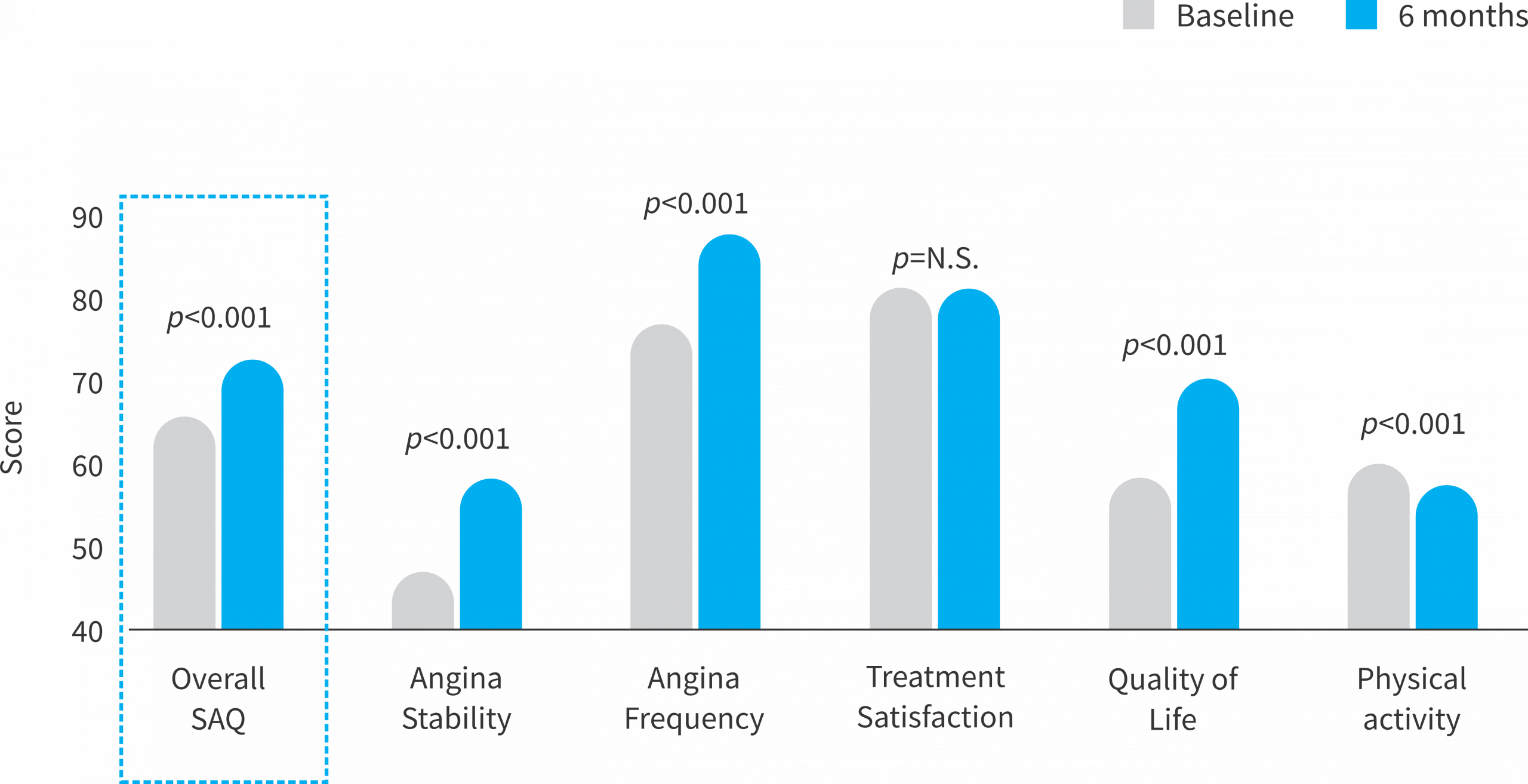
Overall, a strong symptomatic improvement is detected, mostly driven by better quality of life and less symptomatic burden.
Absolute differences in SAQ between baseline and 6 months with most pronounced effects in high complexity CAD-patients
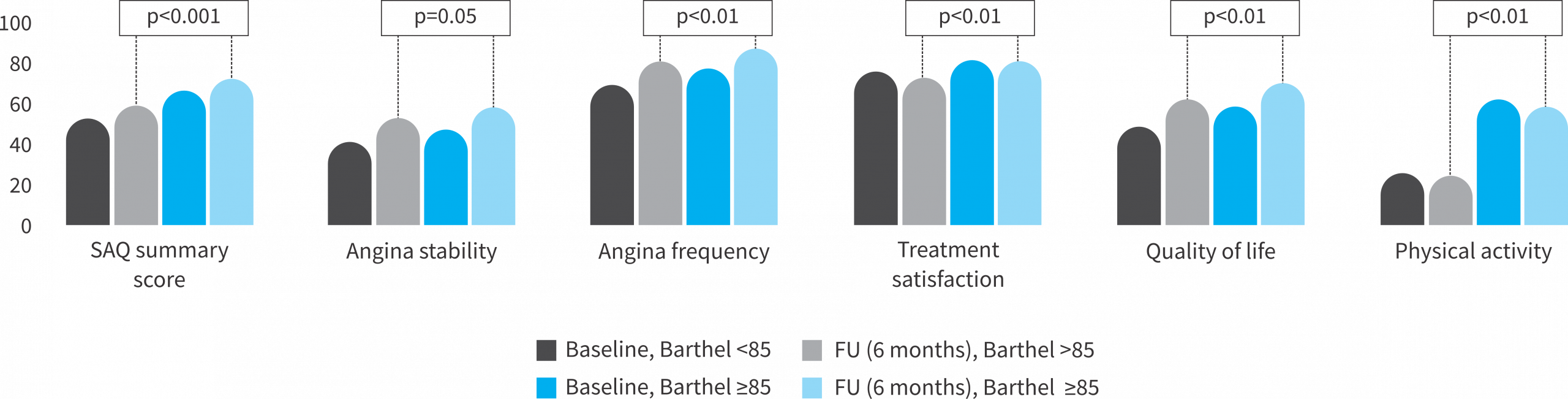
Comparison of patient reported outcomes (PROM) among different frailty categories: Quality of life and symptomatic burden
➔ Barthel index 85 vs. >85
➔ Timed Up & Go ≥11s vs. <11s
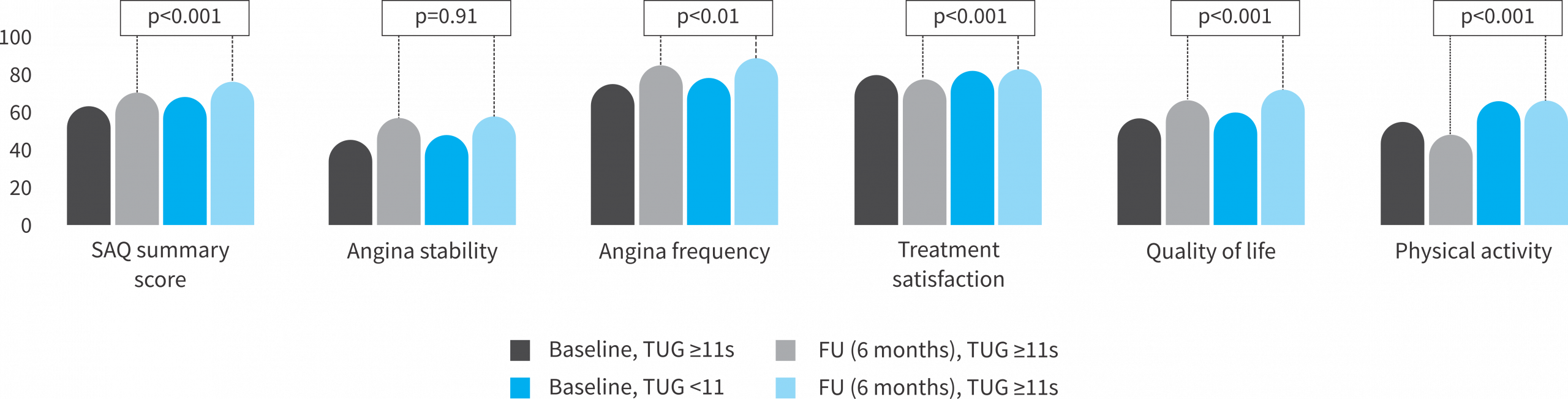
- The frailer, the most symptomatic patients @ baseline
- The frailer, the most benefit in QoL and angina symptom burden
Conclusions:
- Largest prospective PCI cohort in the elderly evaluating both safety endpoints and patient-reported outcomes (PROMs) for PCI using with the newest generation Supraflex Cruz stent platform.
- PCI using Supraflex Cruz with excellent technical performance and consistent procedural success within this highly complex patient group.
- Comparable event rates to previous lower-risk SES PCI cohorts, confirming the strong safety profile of Supraflex Cruz platform in a challenging aged PCI-all-comer population.
- Marked improvement in QoL following PCI with Supraflex Cruz reflecting meaningful clinical benefit in this vulnerable cohort of octo- and nonagenarian with highest improvement of both symptom- and overall disease burden within the frailest patients.
Baseline Characteristics
| Category | All patients (n = 1993) |
|---|---|
| Female (%, n) | 38.2 % (762) |
| Age (mean±SD) | 84.3 (3.1) |
| BMI (mean±SD) | 26.2 (4.2) |
| Hypertension (%, n) | 57.1 % (1138) |
| Diabetes Mellitus (%, n) | 28.9 % (575) |
| Dyslipidemia (%, n) | 63.6% (1268) |
| Positive family history (%, n) | 11.9 % (237) |
| Previous MI (%, n) | 19.1 % (381) |
| Previous PCI (%, n) | 42.3 % (843) |
| Previous CABG (%, n) | 150 (7.5%) |
| Known PAD (%, n) | 14.0 % (279) |
| COPD (%, n) | 7.8 % (155) |
| Severe CKD (eGFR < 30 ml/min) (%, n) | 6.4 % (128) |
| Hemoglobin < 11 g/dl (%, n) | 13.4 % (267) |
| 3 major bleeding risk factors (BARC) (%, n) | 19.5 % (389) |
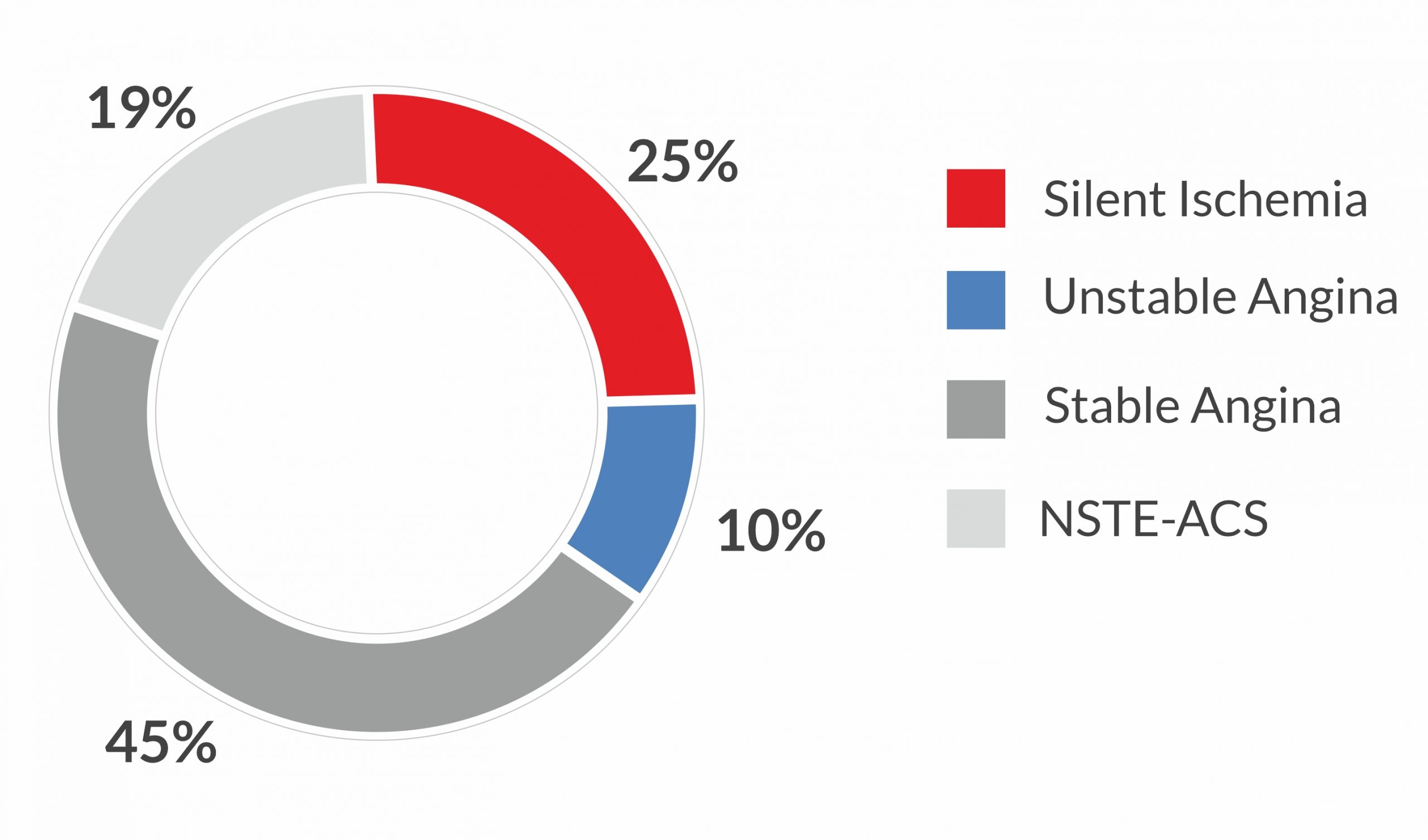
Clinical Presentation
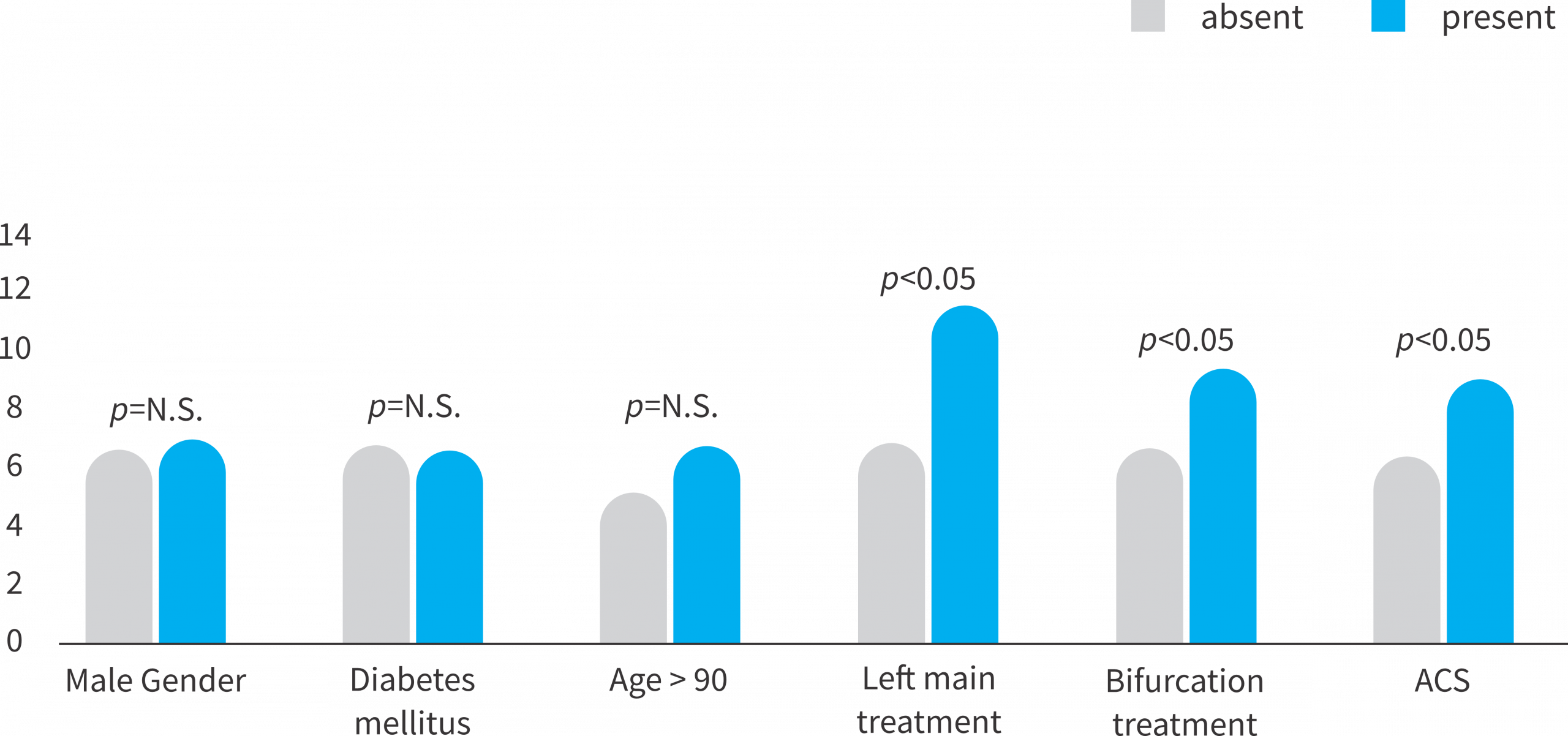
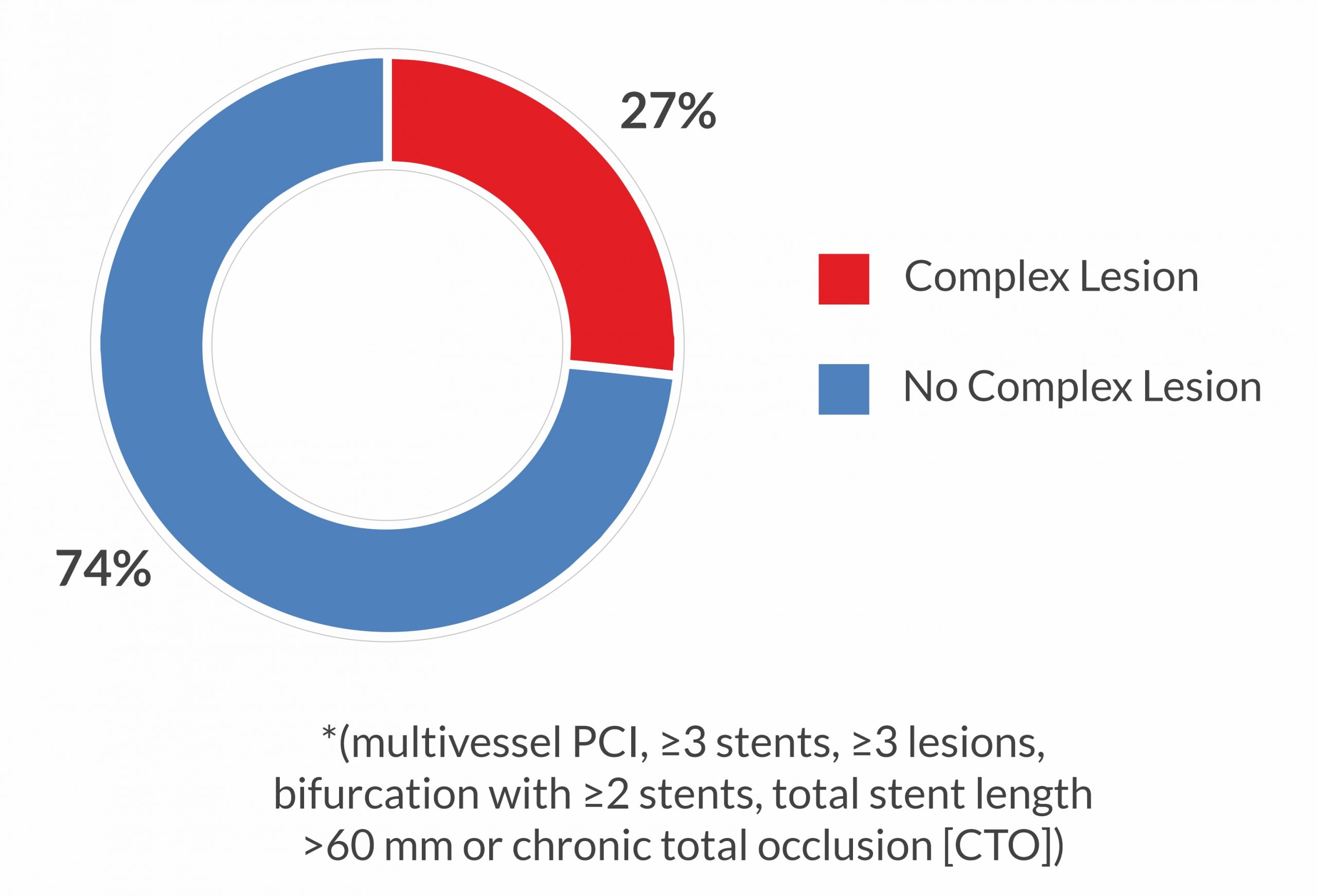
Lesion Complexity*
Stent and Lesion Details
| Device characteristics | All patients (n = 1993) |
|---|---|
| No. of total stents implanted (n) | 3336 |
| No. of stents per patient (mean±𝑆𝐷) | 1.7 ± 0.9 |
| Stent length per patient (mean±𝑆𝐷) | 36.3 ± 22.9 |
| No. of patients with ISR (n; %) | 117 (4.2%) |
| No. of patients with CTO (n; %) | 106 (3.8%) |
| Treatment success | |
| Lesion success* (n; %) | 1988 (99.7%) |
| Procedure success*** (n; %) | 1975 (99.1%) |
| * attainment of < 50 % residual stenosis of the target lesions post-PCI | |
| ** all lesion successfully treated without the occurrence of DOCE during the hospital stay |
Abbreviation: DOCE – Device-Oriented Composite Endpoint, TLR – Target Lesion Revascularization, TV-MI – Target Vessel Myocardial Infarction, CV – Cardiovascular, SAQ – Seattle Angina Questionnaire, SES – Sirolimus-Eluting Stent, PCI – Percutaneous Coronary Intervention, BMI – Body Mass Index, PAD – Peripheral Artery Disease, CABG – Coronary Artery Bypass Grafting, COPD – Chronic Obstructive Pulmonary Disease, CKD – Chronic Kidney Disease, eGFR – Estimated Glomerular Filtration Rate, NSTE-ACS – Non–ST-Elevation Acute Coronary Syndrome